This is the last post on attenuation methods.
Photoelectric effect
There are three main steps in photoelectric effect.
1. A high energy incoming x ray photon knocks out an orbital electron (diagrams show K shell electron being knocked out). The energy of the x ray photon must be equal to or greater than the binding energy of the orbital electron.
2. The electron knocked out of it’s orbit is called a photoelectron. The photoelectron loses its energy as heat in the object being imaged.
3. The atom now has an empty orbital electron. The electrons from other orbitals will jump the shells (i.e. L shell to K shell or M shell to K shell, etc.). This produces characteristic radiation within the object being imaged. This cascade of electrons continues until the atom has filled all it’s empty shells.
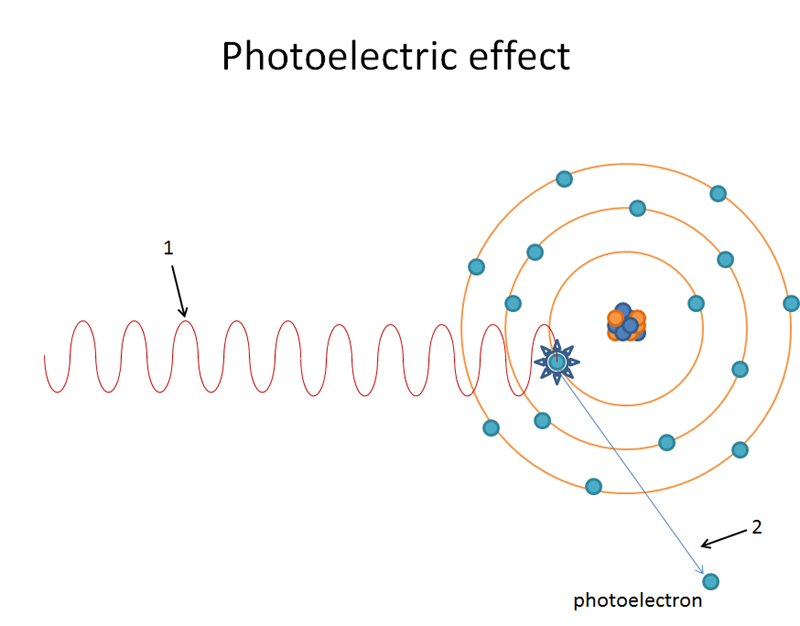 Incoming x ray photon knocks out K shell orbital electron.
Incoming x ray photon knocks out K shell orbital electron.
K shell orbital electron is now a photoelectron.
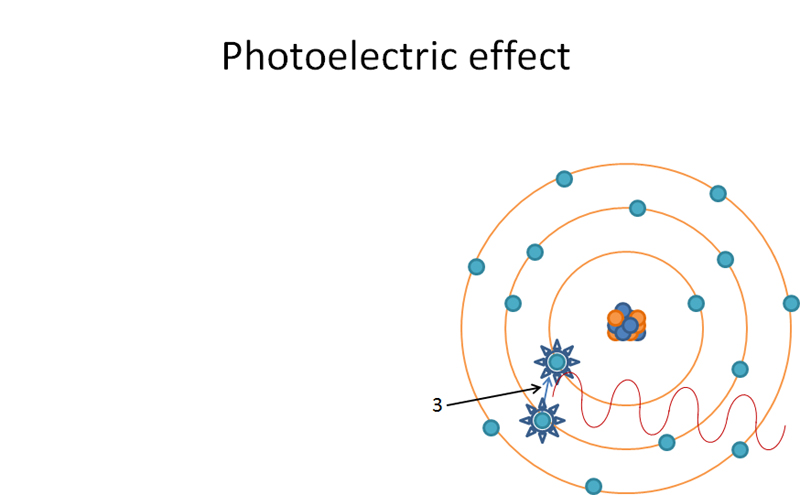 L shell orbital electron ‘jumping orbitals’ and filling in K shell. Produces characteristic radiation when ‘jumping orbitals’.
L shell orbital electron ‘jumping orbitals’ and filling in K shell. Produces characteristic radiation when ‘jumping orbitals’.
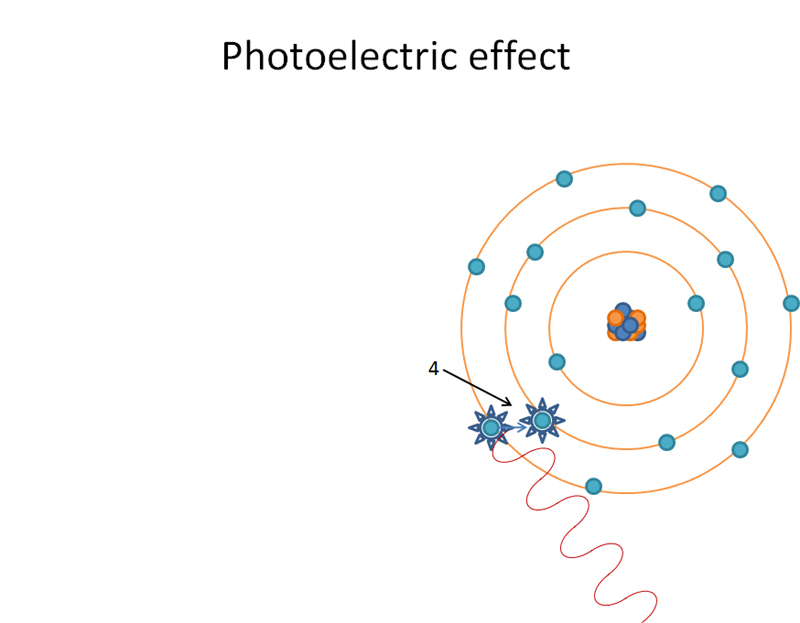 M shell orbital electron ‘jumping orbitals’ and filling in L shell. Produces characteristic radiation when ‘jumping orbitals’.
M shell orbital electron ‘jumping orbitals’ and filling in L shell. Produces characteristic radiation when ‘jumping orbitals’.
The quantity of photoelectric effect occurring within an object is determined by the third power of the atomic number of the absorber. This method of attenuation accounts for approximately 30% of all attenuation.
Should you have any questions about this, please let me know. Thanks and enjoy!
Next week: How was that missed – TRAUMA!

I have a question, don’t s this happen on W atoms or within the radiographic film?
Thank u
Photoelectric effect is similar to characteristic radiation (which occurs in Tungsten atoms). Photoelectric effect does not occur within radiographic film or digital image sensor but in the objects being exposed to x rays (typically teeth and jaws).